Provide leadership and assistance in the development and oversight of institutional processes for the planning, assessment, evaluation and use of feedback for continuous improvement to fulfill the mission and goals of Nebraska Methodist College.
Nebraska Methodist College’s Office of Institutional Effectiveness (OIE) leads, coordinates, and supports initiatives and projects related to strategic, facility and space planning, institutional accreditation and compliance, institutional research, outcomes assessment, sustainability and reporting on the accomplishments of NMC's mission and strategic goals.
OIE strives to align college decision-making, planning and resource allocation processes in support of NMC's mission and student success by fostering a culture of assessment and continuous quality improvement.
IR and Outcomes Assessment collects and analyzes NMC’s quantitative and qualitative data, transforms and disseminates the data into meaningful and actionable information, and supports institutional research and assessment efforts across campus. The IR and Outcomes Assessment team’s focus is on analysis, research, assessment, evaluation and planning across the campus. External reporting remains an important IR function.
NMC Dashboards - Employee Access Only
Trend report of student enrollment, demographics, credit hour loads, quick facts, retention rates, graduation rates, licensure/board pass rates, and program listings for the past year academic years. As well as, an overview of our current staff & faculty.
View Student Enrollment & Achievement Information
The Enrollment Report tracks summary enrollment and demographic information and is created every term.
View the Spring Enrollment Report 2020-2024
View the Fall Enrollment Report 2019-2023
The Annual Retention Report tracks our student retention rates institutionally and per program and drill down by population, status, degrees, division, and department. The Retention Rate is defined as the percent of students enrolling in consecutive fall terms.
The SSI survey is used to assess the level of importance and satisfaction of full-time undergraduate in an on-campus in the areas of Academic Advising, Campus Climate, Recruitment, Financial Aid, Instruction, Campus Support Services, Campus life, Registration and Safety & Security. The results are used to develop action steps to improve the student experience, track progress in these areas and benchmark with other institutions. This is administered every 3 years.
View the Student Satisfaction Inventory (SSI)
The PSOL survey is used to assess the level of importance and satisfaction of online students in distance learning and online programs in the following areas: Academic services, Enrollment services, Institutional perceptions, Instructional services, Student services. The results are used to develop action steps to improve the student experience, track progress in these areas and benchmark with other institutions. This is administered every year.
View the Priorities for Online Learners (PSOL)
This survey is used to assess first-year and senior students Engagement Indicators (EIs), High-Impact Practices (HIPs), and key academic challenge items to illustrate patterns of change or stability. This is administered every 3 years.
View the National Survey of Student Engagement (NSSE)
Our Survey Madness survey is a compilation of questions from all departments to gage our student’s satisfaction with campus life and student services. The results are used to help our efforts to enrich the student experience at NMC. We administer our Survey Madness survey each year in the spring.
View the Survey Madness Student Success, Engagement & Satisfaction Overview
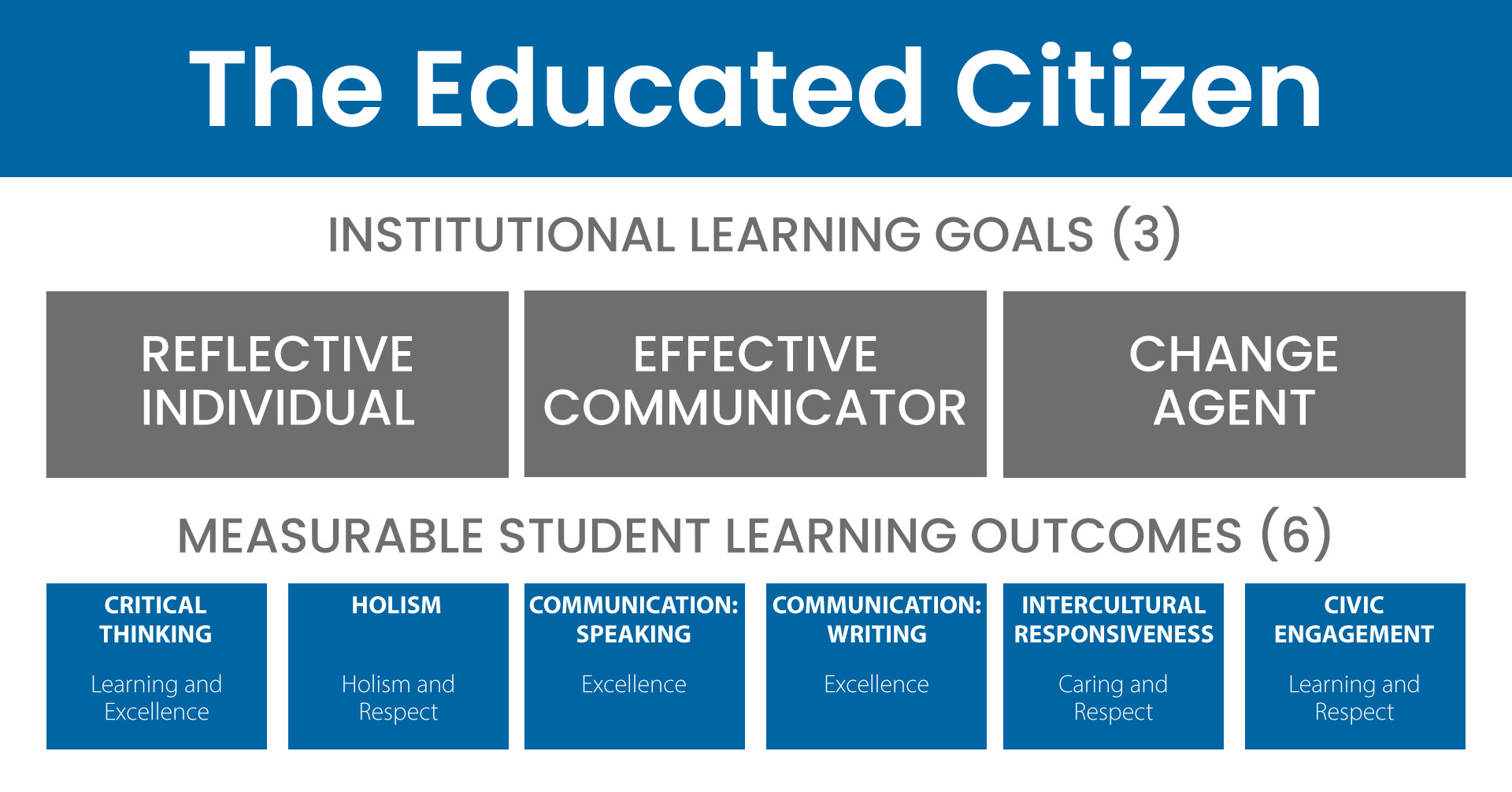
NMC measures student progress toward becoming an effective communicator, a reflective individual, and a change agent.
View the Educated Citizen Student Learning Outcomes Results (PDF)
View the Student Learning Outcomes Assessment Handbook (PDF)
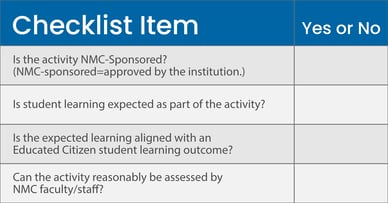
Co-curricular activities are NMC-sponsored learning opportunities that enhance a student’s academic experience. Co-curricular activities are aligned with, and support student development in, Educated Citizen student learning outcomes.
Must answer “yes” to all questions to qualify as co-curricular.
Examples include, but are not limited to: educational workshops, engagement opportunities, student-faculty research, community engagement, peer mentoring programs, and involvement in student organizations.
As a part of our continuous quality improvement effort, we implemented a Culture Audit as a mechanism to help evaluate whether faculty, staff and administration feel that our culture is effective in creating a highly successful organization. The Culture Audit was administered every three years beginning in 1994 and nearly annually since 2016, the audit was revamped to directly measure our mission, core values and culture. The results are reported and action steps are created to address the areas that show the greatest need for improvement. We have worked with external organizations such as Coffman in 2009 and The Pacific Institute in 2018.
Data is most useful when it’s provided as information that can be applied to planning and decision-making. IR & Outcomes Assessment team consults with faculty and staff on formulating goals and objectives, data analysis, research and assessment methods, instrument design, planning and process design, and establishing benchmarks, performance indicators, and learning outcomes. We also provide support in the areas of survey design, formatting and administration using online surveys.
Please use our online form to Submit a Request. You will be notified that your request has been received, assigned and prioritized within 48 hours of being submitted.

OIE oversees and coordinates institutional accreditation activities, monitors the college’s ongoing compliance with accreditation standards, documents student achievement in keeping with federal requirements, and tracks specialized accreditation of college programs.
To ensure institutional effectiveness, OIE facilitates institutional strategic planning, coordinates academic and support unit program reviews, and oversees reporting on assessment of student learning outcomes. The office is also responsible for state and federal institutional compliance reporting.
 NMC maintains full accreditation with The Higher Learning Commission, a regional accreditation agency recognized by the U.S. Department of Education. The College is authorized to offer programs of study leading to certificate, associate, baccalaureate, masters, and doctoral degrees. The last comprehensive evaluation for reaffirmation of accreditation occurred in May 2016. Learn more about program specific accreditation.
NMC maintains full accreditation with The Higher Learning Commission, a regional accreditation agency recognized by the U.S. Department of Education. The College is authorized to offer programs of study leading to certificate, associate, baccalaureate, masters, and doctoral degrees. The last comprehensive evaluation for reaffirmation of accreditation occurred in May 2016. Learn more about program specific accreditation.
The College is on what the commission calls the Open Pathway. The Open Pathway program is one of two options that an institution has for maintaining its accreditation with HLC. The Open Pathway's 10-year cycle focuses on quality assurance and institutional improvement appropriate to the College Mission. In the final year of the cycle, the institution undergoes a comprehensive evaluation to ensure that it is meeting the Criteria for Accreditation, pursuing institutional improvement and complying with certain requirements set by the U.S. Department of Education.
HLC member institutions are required to notify HLC of changes in accordance with the substantive change policy and, when required, seek approval prior to the initiation of changes. Member institutions are required to have policies and procedures to ensure that all substantive changes are reported to the Commission in a timely manner.
The Nebraska Methodist College Consumer Information Directory is designed as a quick reference tool for College personnel, students, prospective students, and other consumers. Included in this directory is consumer information as required by the Higher Education Act and the Public Health Service Act. The Higher Education Opportunity Act (Public Law 110-315) (HEOA) was enacted on August 14, 2008, and reauthorizes the Higher Education Act of 1965, as amended (HEA). Information contained in this directory is updated annually.

The Nebraska Methodist College (NMC) Institutional Review Board (IRB) is a federally registered board responsible for the protection of human subject research at NMC. The IRB performs critical oversight in the review, approval and monitoring of all research protocols, quality improvement projects and evidenced-based practice projects by students and faculty at NMC.
External requests to recruit faculty, staff or students to participate in online surveys or other research protocols must submit application materials to the NMC IRB for review and must obtain NMC IRB approval prior to conducting research. All external research/projects must be in alignment with the mission, vision, core values and strategic priorities of Nebraska Methodist College.
To ensure protection of human subjects, all investigators shall be familiar with:
Inquiries can be submitted to the IRB Chair at IRB@methodistcollege.edu.

About: NMC’s facilities management and space planning provides campus support for services relating to campus buildings, labs & classrooms, campus housing, grounds and the overall campus environment, which includes planning, constructing, managing improvements and maintenance. NMC’s facility management and space planning is accomplished through coordination with Methodist Health System and ensures NMC’s operates and maintains a physical environment that supports NMC students, faculty, staff, and visitors.
Capital Projects:
2020
OT/PTA Lab Construction—completed fall 2020
Leinart Lobby Renovation—completed spring 2021
A&P Lab Tech Upgrade—completed winter 2020
2021
Clark Lobby/Dining Update—completed summer 2021
Clark Patio Refresh - completed summer 2021
Nursing Lab High-Fidelity Simulators Upgrades—completed summer 2021 and 2022
Testing Center - completed fall 2022
2022
John Moritz Library and Learning and Academic Resource Commons (LARC) remodel - completed spring 2022
Wellness Engagement (WE) Center - completed summer 2022
Classrooms 3214/3216/3218 Teaching Technologies upgrades - in progress
Student Leader Wall - planning
Sustainability
.jpg?width=284&name=receptacles%20(2).jpg)
Waste Diversion: In 2020, NMC received a Recycling Equipment Grant from the Nebraska Recycling Council, in conjunction with the Nebraska Wildlife Trust. The Trust is funded by proceeds from the Nebraska Lottery and has awarded more than $289 million to over 2,100 natural resource projects in Nebraska since 1993. The Nebraska Recycling Council is a non-profit, membership organization of public and private organizations, as well as individuals, and has been a recycling advocate for Nebraska since 1980. The funding from this grant allowed NMC to purchase new recycling receptacles for all buildings and housing units on the Josie Harper Campus.
In summer of 2021, NMC piloted a successful composting program on campus in partnership with Hillside Solutions. NMC is proud to report that all paper towel waste from campus restroom facilities is now being composted. Additional composting receptacles are located in the Clark Dining areas to divert food waste. Future expansion of the composting program to campus housing is forthcoming.
Deja Brew: In 2020, Deja Brew phased out all Styrofoam food and drink containers and transitioned to recyclable & compostable containers. This includes clamshells, food containers and drinking cups.